We Work in Quality,
Not Volume
Our expertise is defined by a personalized
experience to help those wronged find justice
IVC Filter
Patients who are at risk of developing blood clots, but who are not candidates for blood thinner medication, often have IVC (inferior vena cava) filters implanted, especially after major surgery.
These small, cage-like or spider-like devices are implanted in the inferior vena cava—the main vessel moving blood from the lower body to the heart. The IVC filter’s purpose is to prevent blood clots from entering the heart, lungs, kidneys or brain, by catching the clots in the bloodstream and allowing them to break down over time.
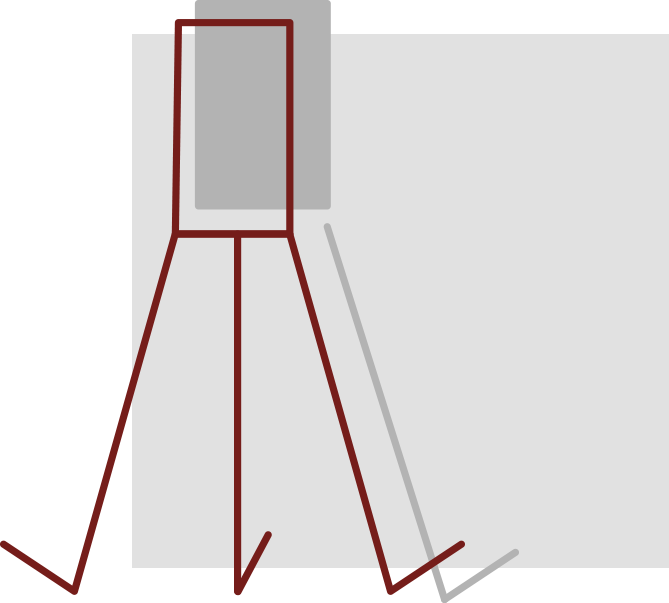

IVC Filter
Patients who are at risk of developing blood clots, but who are not candidates for blood thinner medication, often have IVC (inferior vena cava) filters implanted, especially after major surgery.
These small, cage-like or spider-like devices are implanted in the inferior vena cava—the main vessel moving blood from the lower body to the heart. The IVC filter’s purpose is to prevent blood clots from entering the heart, lungs, kidneys or brain, by catching the clots in the bloodstream and allowing them to break down over time.
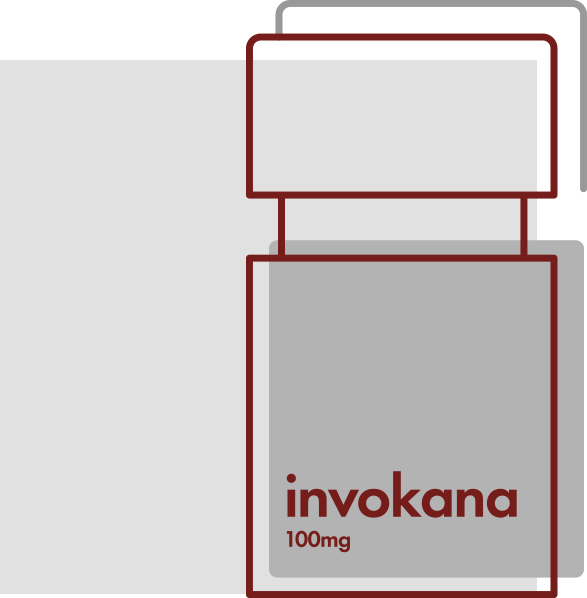
Invokana Drug
Invokana (canagliflozin) and Invokamet (canagliflozin/metformin) are SGLT2 inhibitors that were developed by Mitsubishi Tanabe Pharma Corporation for more than a decade before being licensed out to Janssen Pharmaceuticals, a division of Johnson & Johnson.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a group of drugs FDA-approved to treat low blood sugar in adults with type 2 diabetes, in conjunction with diet and exercise. Invokana was formally approved by the FDA in March 2013 to improve glycemic control in adults with type 2 diabetes. It was the first SGLT2 inhibitor approved for use in the United States. The prescription medication works by inhibiting SGLT2, a carrier responsible for the reabsorption of glucose back into the bloodstream. By stopping the carrier, less glucose is reabsorbed by the kidneys, and more glucose is lost during urination. This ultimately leads to lower blood glucose levels in those with type 2 diabetes.
Hernia Mesh implants
A hernia occurs when an organ, intestine or fatty tissue squeezes through a hole or a weak spot in the surrounding muscle or connective tissue. Hernias often occur at the abdominal wall. Sometimes a hernia can be visible as an external bulge particularly when straining or bearing down.

Hernia Mesh implants
A hernia occurs when an organ, intestine or fatty tissue squeezes through a hole or a weak spot in the surrounding muscle or connective tissue. Hernias often occur at the abdominal wall. Sometimes a hernia can be visible as an external bulge particularly when straining or bearing down.
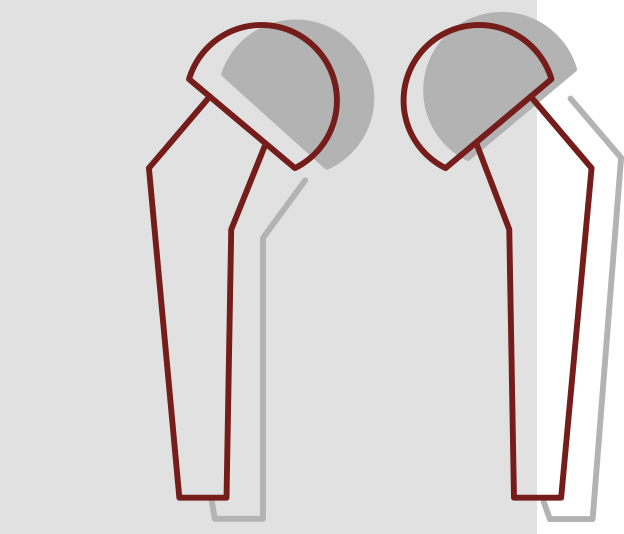
Hip Implant Failures
Hip implants are medical devices intended to restore mobility and relieve pain usually associated with arthritis and other hip diseases or injuries. Every hip implant has a distinct set of benefits and risks. The key design features of each implant including size, material and dimensions make each system unique. In addition, the same hip implant system will have different outcomes in different patients. It is also important to recognize that hip implants may need to be replaced eventually. Factors that influence the longevity of the device include the patient’s age, sex, weight, diagnosis, activity level, conditions of the surgery, and the type of implant chosen.
Attune Knee Implant Failure
DePuy Synthes is the medical devices unit of Johnson & Johnson. In June of 2015, the U.S. Food and Drug Administration issued a worldwide voluntary recall of several of the component parts of its Attune knee replacement system, for failing to receive prior FDA approval. This recall affects approximately 3,500 knee units. In their warning letter to the company, the FDA found that these components had not received approval and additionally, had been altered and were no longer safe. These components had not gone through the more stringent approval process of the FDA. Many times, a devise and components will obtain approval through a minimum requirement called the 510k process, because they resemble existing components already on the market.

Attune Knee Implant Failure
DePuy Synthes is the medical devices unit of Johnson & Johnson. In June of 2015, the U.S. Food and Drug Administration issued a worldwide voluntary recall of several of the component parts of its Attune knee replacement system, for failing to receive prior FDA approval. This recall affects approximately 3,500 knee units. In their warning letter to the company, the FDA found that these components had not received approval and additionally, had been altered and were no longer safe. These components had not gone through the more stringent approval process of the FDA. Many times, a devise and components will obtain approval through a minimum requirement called the 510k process, because they resemble existing components already on the market.

